Design, synthesis, and biological activity of substituted 2-amino-5-oxo-5H-chromeno[2,3-b]pyridine-3-carboxylic acid derivatives as inhibitors of the inflammatory kinases TBK1 and IKK epsilon for the treatment of obesity.
Beyett, T.S., Gan, X., Reilly, S.M., Gomez, A.V., Chang, L., Tesmer, J.J.G., Saltiel, A.R., Showalter, H.D.(2018) Bioorg Med Chem 26: 5443-5461
- PubMed: 30270002
- DOI: https://doi.org/10.1016/j.bmc.2018.09.020
- Primary Citation of Related Structures:
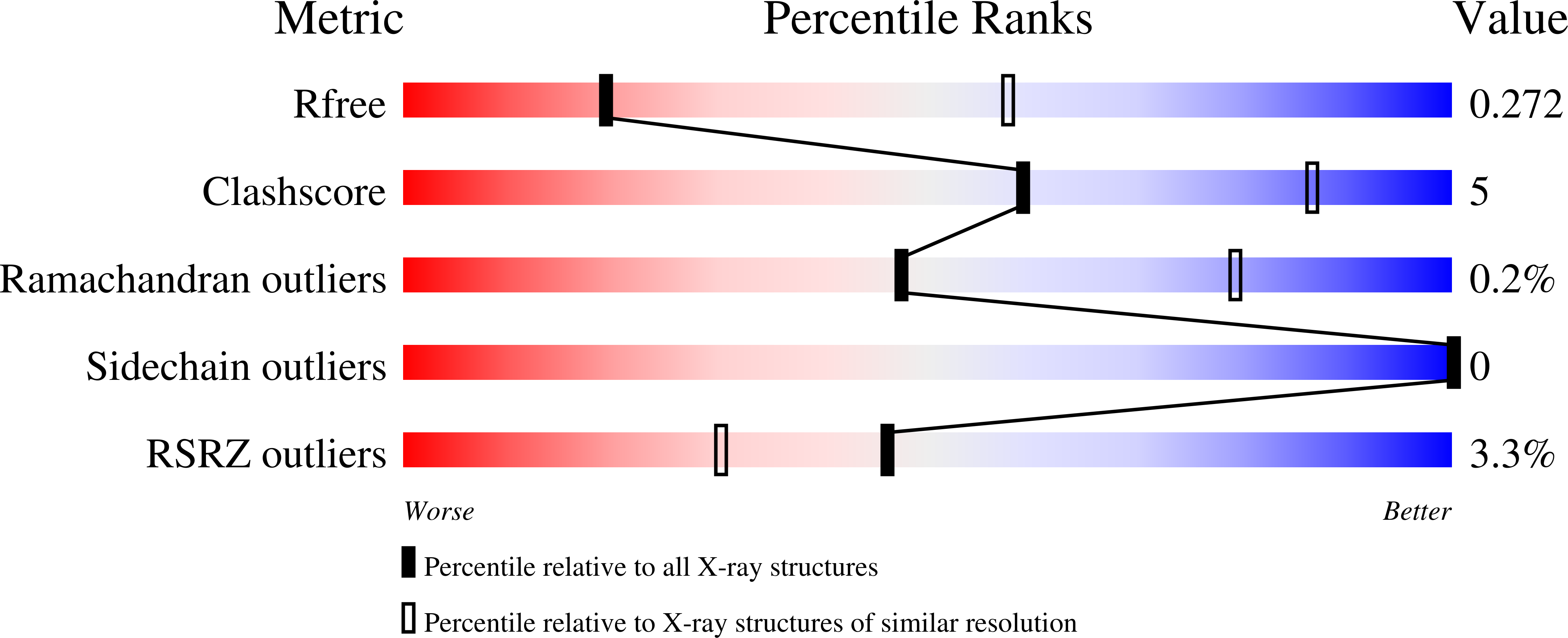
6CQ0, 6CQ4, 6CQ5 - PubMed Abstract:
The non-canonical IκB kinases TANK-binding kinase 1 (TBK1) and inhibitor of nuclear factor kappa-B kinase ε (IKKε) play a key role in insulin-independent pathways that promote energy storage and block adaptive energy expenditure during obesity. Utilizing docking calculations and the x-ray structure of TBK1 bound to amlexanox, an inhibitor of these kinases with modest potency, a series of analogues was synthesized to develop a structure activity relationship (SAR) around the A- and C-rings of the core scaffold. A strategy was developed wherein R 7 and R 8 A-ring substituents were incorporated late in the synthetic sequence by utilizing palladium-catalyzed cross-coupling reactions on appropriate bromo precursors. Analogues display IC 50 values as low as 210 nM and reveal A-ring substituents that enhance selectivity toward either kinase. In cell assays, selected analogues display enhanced phosphorylation of p38 or TBK1 and elicited IL-6 secretion in 3T3-L1 adipocytes better than amlexanox. An analogue bearing a R 7 cyclohexyl modification demonstrated robust IL-6 production in 3T3-L1 cells as well as a phosphorylation marker of efficacy and was tested in obese mice where it promoted serum IL-6 response, weight loss, and insulin sensitizing effects comparable to amlexanox. These studies provide impetus to expand the SAR around the amlexanox core toward uncovering analogues with development potential.
Organizational Affiliation:
Program in Chemical Biology, University of Michigan, Ann Arbor, MI 48109, United States; Life Sciences Institute, Departments of Pharmacology and Biological Chemistry, University of Michigan, Ann Arbor, MI 48109, United States.